Mass spectrometry has evolved into the gold standard for proteome research because of its speed, throughput and accuracy of collecting both quantitative and qualitative data on the analytes of interest. However, much of its processing power on complex samples in discovery settings can be attributed to prior separation using liquid chromatography and, in the case of proteomics, often reversed phase liquid chromatography (RPLC).
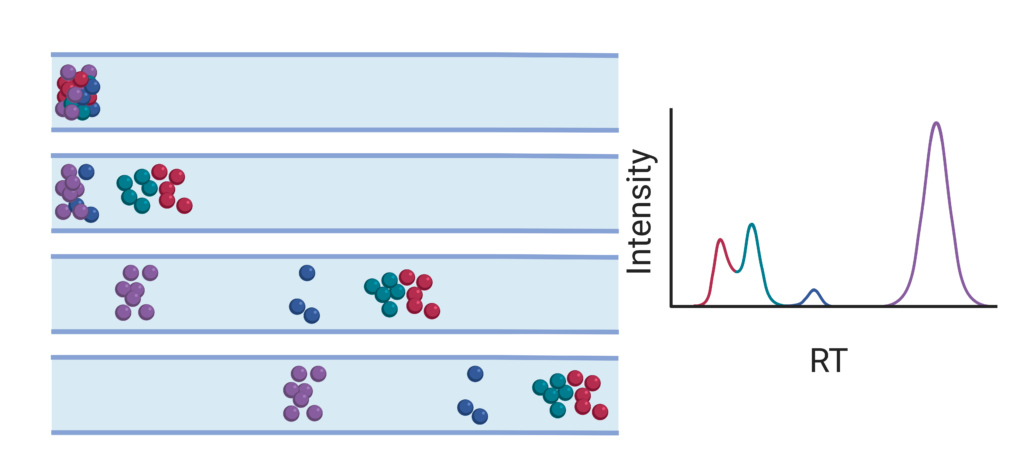
In common shotgun or bottom-up proteomics workflows, peptides are first separated based on their hydrophobicity – which is a product of their amino acid composition – using RPLC. To this end, the peptides are dissolved in an aqueous mobile phase, which ensures retention on the hydrophobic stationary phase of the analytical column, usually C18 bonded silica particles. The individual analytes or pepti
des migrate at different velocities along the column in the mobile phase depending on their distribution between the mobile- and stationary phase. In general, longer peptides have a higher hydrophobicity, and thus a higher affinity for the stationary phase due to the carbon backbone, resulting in a lower velocity and higher retention time (tR). To obtain faster and better separation, gradient elution is applied. Herein the aqueous mobile phase is gradually spiked with organic mobile phase, thus eluting peptides over time according to increasing hydrophobicity or affinity for the stationary phase. Due to the distribution between both phases and a differing total path length through the particles, elution profiles have a normal distribution over time, which can be used to characterize the performance of the LC-system.
At ProGenTomics we use ultra high performance liquid chromatography systems (UPLC), e.g. the Waters’ M-class acquity that can handle pressures over 1.000 bar (roughly 15.000 psi). These instruments are capable of running both in nanoflow (<500nl/min) and capillary flow (1-20µL/min). Nanoflow can obtain the highest sensitivity due to optimal ionisation in the electrospray ion source of our instruments. However, it often lacks robustness and stability, which makes it less suited for quantitative analyses and large experiments. Capillary flow on the other hand is less sensitive, but is capable of analysing for days on end without intervention and therefore suited for large experiments and quantitative analyses.