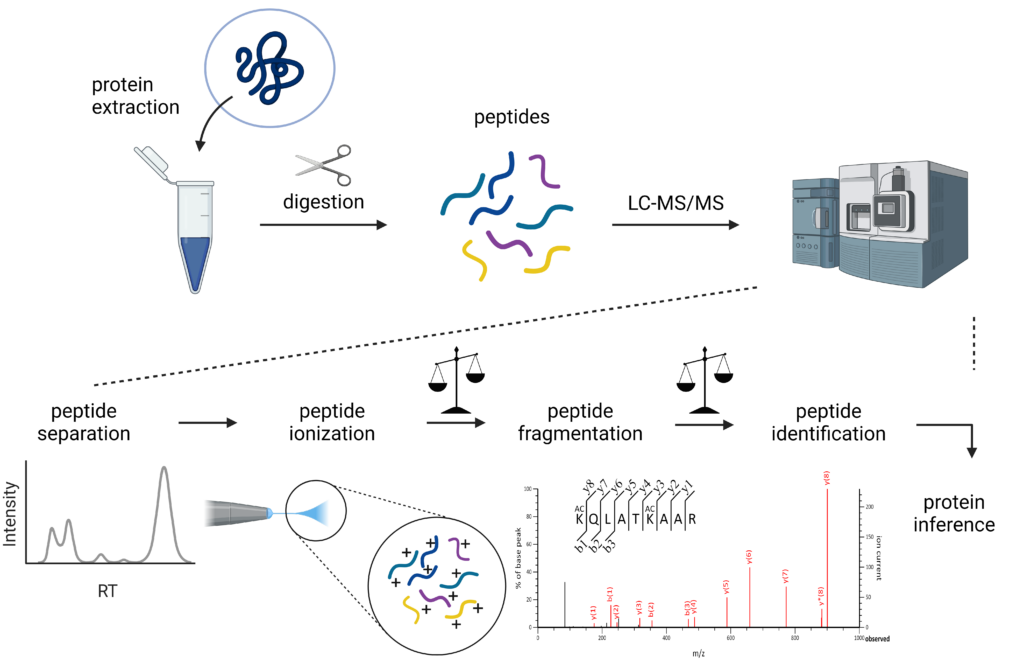
“Proteomics” comprises the large-scale comprehensive study of the proteome, being the entire protein complement expressed by the genome of a or multiple cell(s) or organism(s). The main aim of a proteomics study is often the quantitative and/or qualitative comparison of the protein content of samples (in order to find differences between e.g. healthy and diseased or treated and control). In traditional MS-based proteomics, proteins are often digested with trypsin to generate smaller peptides, so-called bottom-up proteomics. Trypsin specifically and efficiently cleaves at the C-terminal end of arginine and lysine, which has several advantages; (I) peptides generated are easily ionized because of the presence of arginine or lysine at the C-terminal end, (II) they usually have a double or triple charge, making it easy to resolve the isotope envelope in the mass spectrum and separate multiple charged signal from singly charged noise, and (III) they are readily separated by reversed phase liquid chromatography. Intact protein analysis or top-down proteomics, i.e. without digestion into peptides, can be performed on less complex samples and is often used to characterize biologicals and biopharmaceuticals.